History of atomic theory pdf
History of atomic theory pdf
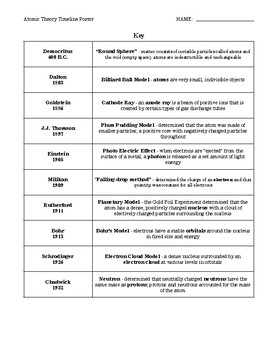
Democritus proposes describes the 1st atomic theory 460 – 370 BC History of the Atom – Timeline Antoine Lavoisier makes a substantial number of contributions
making the foundation of modern atomic theory of matter. * the idea that everything is made of distinct atoms has been a subject of skeptical discussions as recently as the beginning of the twentieth century, before Einstein’s observation of Brownian motion in 1905 and Max von Laue’s observation of the diffraction of X-rays by crystals in 1912 provided strong support for the atomistic
This is a great cut and paste resource for the ‘history of atomic theory’ topic. Students match an image with the model and scientist name, then with a description of the model.
The History of the Atom Timeline: 400 BC He called this the theory of the universe: All matter consists of atoms, which are bits of matter too small to be seen. There is an empty space between atoms Atoms are completely solid Atoms have no internal structure Each atom (of a different substance) is different in size, weight and shape. Timeline: 1800’s Scientist: John Dalton John Dalton
michael faraday atomic theory Atomic Structure Timeline – Cool Periodic Table – History of the Atom. Scientists and Their Contribution to the Model
Atomic theory is a scientific description of the nature of atoms and matter that combines elements of physics, chemistry, and mathematics. According to modern theory, matter is made of tiny particles called atoms, which are in turn made up of subatomic particles.
history of atomic theory. The process of science is obvious throughout several centuries as these The process of science is obvious throughout several centuries as these scientists developed a deeper understanding of what the atom looks like.
Atomic Theory The History of the Atom – Student Worksheet Directions: each student in the group will answer the questions below in their own words and in complete
theory that matter was made of four “elements”: earth, fire, water, air. •Aristotle was wrong. However, his theory persisted for 2000 years. fire air water earth •This small particle he called “atomos”. 1808 -Dalton proposed a modern atomic model based on experimentation not on pure reason. •All matter is made of atoms. •Atoms of an element are identical. •Atoms of different
The History of Atomic Theory The Development of Atomic Theory Part 1: Democritus to Thomson What is an Atom? An atom is the smallest particle that an …
1 A Brief History of Atomic Theory The “modern” atomic picture has evolved over many centuries Often fraught with religious and philosophical overtones History of the Atom Project – LZ95
•English chemist & physicist •1766-1844 • Considered to be the father of our modern atomic theory John Dalton
English chemist and physicist John Dalton extended Proust’s work and converted the atomic philosophy of the Greeks into a scientific theory between 1803 and 1808. His book A New System of Chemical Philosophy ( Part I , 1808; Part II , 1810) was the first application of atomic theory to chemistry.
History of Atomic Theory – Free download as Word Doc (.doc), PDF File (.pdf), Text File (.txt) or read online for free.
Webquest Atomic Theories and Models

History of the Atomic Theory Chemistry LibreTexts
Activity – Atomic Theory Foldable . Objectives . 1. To trace the development of the atomic model. 2. To recognize the different atomic models developed. Materials . Colored Paper printed with Title Information and one sheet of white paper . Colored pencils . Handout with Discovery Timeline . Procedure – Outside of Foldable. 1. Watch teacher to see how to place the white paper on the inside
Book:History of Atomic Theory. Jump to navigation Jump to search. WARNING! The Book Creator software has significant limitations. Book creation may be disabled while a replacement is adapted and installed. An alternative open source is available, see MediaWiki2LaTeX. For Help with downloading a Wikipedia page as a PDF, see Help:Download as PDF. This is a Wikipedia book, a collection of
atomic characteristics. romF their sizes to their spectra, much was known about atoms, but little had been explained in terms of the laws of physics. Bohr’s theory explained the atomic …
The history of atomic theory is an excellent example of how mankind struggled to understand their world prior to the scientific process, and how the scientific process enabled civilization to make many scientific discoveries and technological advancements.
Chemistry Lessons Teaching Chemistry Science Chemistry Physical Science Science Education Chemistry Review Chemistry Classroom High School Chemistry Atomic Theory Forward Build an Atom—this is cool.really shows how protons, neutrons and electrons are added to create a new element.
History of the OBJECTIVES Understand the law of definite proportions. Define a scientific law and identify how observations become a law. Explain that a scientific theory is …
A Brief History of Atomic Theory. This Section will focus on Scientists who have had an impact on the study of the atom. 470-380 B.C. Democritus proposed that matter cannot be broken down indefinitely.
Dalton’s atomic theory agrees with modern atomic theory except for the statement that a. atoms are not divided in chemical reactions. b. all atoms of the same element have the same mass.
In chemistry and physics, atomic theory is a scientific theory of the nature of matter, which states that matter is composed of discrete units called atoms. It began as a philosophical concept in ancient Greece and entered the scientific mainstream in the early 19th century when discoveries in the field of chemistry showed that matter did indeed behave as if it were made up of atoms. The word
What unexpected thing happened that suggested Thomson was not correct about the structure of atoms? _____ Why did some of the alpha particles shot at the gold not

Name the date and inventor of the modern version of the Atomic Theory 4. I was born in 1831 and showed that electricity and magnetism are scientifically related.
PROBLEM (PageIndex{6}) Predict and test the behavior of α particles fired at a “plum pudding” model atom. (a) Predict the paths taken by α particles that are fired at …
View Webquest_-_History_of_Atomic_Theory_X.docx from MWH 101 at Valley Forge High School. Webquest: The History of the Atomic Model Read the heading …
atomic history.pdf – The History of the Atomic Theory Fill in the boxes for each scientist who assisted the development of the atomic theory. John Dalton His Theory: A picture to describeit:.

The Atomic Theory The idea that the elements are capable of being split up into very tiny particles was rst evolved by the Greeks. The word atom comes from the greek word atomos which means indivisible and the idea that the elements are made up of atoms is called the atomic theory. A scienti c theory is a scienti c idea which has been accepted by scientists after due consider- ation. When an
The atomic theory is developed since 2000 years ago the Greek philosopher Democritus proposed that there was a limit to how small one could be divide matter, this smallest matter indivisible particle was called “atom”. However this atomic theory of Democritus was criticized by Aristotle who proposed a model based on four basic “elements” of earth, air, fire and water.
History of Atomic Theory. Science 16 Sep 1966: Vol. 153, Issue 3742, pp. 1370-1371 DOI: 10.1126/science.153.3742.1370-a . Article; Info & Metrics; eLetters; PDF; This is a PDF-only article. The first page of the PDF of this article appears below. Science. Vol 153, Issue 3742 16 September 1966 . Table of Contents ; Print Table of Contents ; Back Matter (PDF) Ed Board (PDF) Front Matter (PDF
A Brief History of Atomic Theory – Relatively Interesting
1 1 A Brief History of Atomic Theory The “modern” atomic picture has evolved over many centuries Often fraught with religious and philosophical overtones
An atom is the smallest particle that an element can be divided and still be that element. For example the smallest particle of carbon is a single
The Atomic Theory in Culture. The atomic, microscopic way of looking at matter is actually a fairly new development. The United States has already celebrated its two-hundredth birthday, whereas the atomic theory is only about 175 years old.
History of Dalton’s Atomic Theory. Although the concept of the atom dates back to the ideas of Democritus, the English meteorologist and chemist John Dalton formulated the first modern description of it as the fundamental building block of chemical structures. – history of bengali literature pdf 4/11/2013 · The History of Atomic Chemistry: Crash Course Chemistry #37 in terms of Atomic Chemistry, Hank takes us on a tour of the folks that were part …
1803 Dalton’s Modern Atomic Theory 1. Matter is made up of atoms that are indivisible and indestructible. 2. All atoms of an element are identical.
We’ve condensed 2,500 years of atomic theory into a single infographic.
History of the atomic theory essay Our upgrade to newer machines policy assures you will have up-to-date machines with low hours and new technology. Combined with this and our 24 hour breakdown service, our well maintained plant will be sure to keep your operations moving forward.
2.1 Historical Development of Atomic Theory. 2 2.1.1 The Periodic Table of the Elements 2.1.2 Discovery of Subatomic Particles & the Bohr Atom Each element emits light of specific energies when excited by electric discharge or heat. For the H-atom (Balmer, 1885): 6 5 4 3 2 n = 3 2.1.2 Discovery of Subatomic Particles & the Bohr Atom Johann Jacob Balmer (Physicist, 1825 – 1899) The Hydrogen
John Dalton is now called the father of modern atomic theory for his efforts. His atomic theories were introduced in 19th century England. In September of 1803, John Dalton wrote his first table of atomic weights in his daily logbook. Two years after he developed his atomic weights, he published them in a book called “A New System of Chemical Philosophy”. In it he was the first to propose
Page 1 of 5. Mark Important Points in Margin Date: _____ Use this space for additional notes. Chemistry I Page 1 of 5 History of Atomic Theory
HISTORICAL OUTLINE of the Atomic Theory and the Structure of the Atom . Development of the Atomic Theory . Democritus (460-370 BC) First proposed the existence of an ultimate particle. Used the word “atomos” to describe this particle. Democritus . Aristotle (384-322 BC) was a proponent of the continuum. He believed in the four elements of air, earth, water and fire. Aristotle felt that
02 – Evolution of the Atomic Model During this portion of the Chemistry Unit, students will learn what the atom is, our current theory of what it looks like, and how our views of it have evolved over time.
history of atomic theory Google Search Chemistry
The atomic nucleus was discovered in the gold foil experiment conducted by Geiger and Marsden under the supervision of Ernest Rutherford in 1909. Important Atom Facts All …
DUE DATE: _____ Name: _____ History of the Atom Project The atomic theory of matter is an excellent illustration of the process of science.
A Brief History of Atomic Theory Dalton’s Atomic Theory The “modern” atomic picture has evolved over many centuries. Often fraught with religious and philosophical overtones.
History of Atomic Theory Around 400 BC, a Greek philosopher named Democritus suggested the first atomic theory, explaining that all things are “composed of minute, invisible, indestructible particles of
The History of the Atom Socorro Independent School District

2.1 A History of Atomic Theory (Problems) Chemistry
To them,human reasoning was superior to experimentation. Democritus used an example of a beach to support his theory. From afar,the beach appears to be a solid mass.
the atomic number can tell you the number of electrons as well if the atom is neutral (no charge). The atomic mass is the weighted average of the mass numbers of all isotopes (isotopes have the same number of protons, but a different number of neutrons) of an element found in nature.
ScientificTheory • Summarizes a hypothesis or group of hypotheses that have been supported with repeated testing • If enough e…
This can be explained by atomic theory if the copper-to-chlorine ratio in the brown compound is 1 copper atom to 2 chlorine atoms, and the ratio in the green compound is 1 copper atom to 1 chlorine atom. The ratio of chlorine atoms (and thus the ratio of their masses) is therefore 2 to 1 (Figure (PageIndex{4})).
2007 – Douglas Gilliland Honors Physical Science @ Sarasota High A Brief History of Atomic Theory The man of science does not want to discover in order to know – he wants to know in order to discover.
Atomic Theory Summary DBooth.net

A Brief History of Atomic Theory birdvilleschools.net
MHS Chemistry Atomic Theory. Throughout history, human beings have wondered what they would see if they could just look a little closer. Looking outward from Earth, this has led to the exploration of our solar system directly, and of the rest of the entire universe through various telescopes.
All matter is made up of atoms, the basic unit of a chemical element. This is something we now take as a given, and one of the things you learn at the beginning of high school or …
Dalton’s Atomic Theory: John Dalton (1766 – 1844) was born into a modest Quaker family in England. He earned his living for most of his life as a teacher and public lecturer.
N B.1 The History of Atomic Theory.pdf – Google Drive Main menu
History of Atomic Theory. Picture an atom. What does it look like? Most likely it will resemble something like this: a fairly large nucleus surrounded by orbiting electrons whizzing around the
History of Atomic Theory Science


The History of Atomic Theory Mr. Finke’s Science Class
A Brief History of Atomic Theory ThoughtCo
– The History of Atomic Theory mrhee.weebly.com
History of Atomic Theory woburnpublicschools.com
/spectrum-wave-154957117-57d009f73df78c71b621f3d8.jpg)
2.1 A History of Atomic Theory Chemistry LibreTexts
A Brief History of Atomic Theory msjilesen.yolasite.com
2.1 A History of Atomic Theory (Problems) Chemistry
Activity – Atomic Theory Foldable
View Webquest_-_History_of_Atomic_Theory_X.docx from MWH 101 at Valley Forge High School. Webquest: The History of the Atomic Model Read the heading …
An atom is the smallest particle that an element can be divided and still be that element. For example the smallest particle of carbon is a single
History of Atomic Theory. Picture an atom. What does it look like? Most likely it will resemble something like this: a fairly large nucleus surrounded by orbiting electrons whizzing around the
The atomic nucleus was discovered in the gold foil experiment conducted by Geiger and Marsden under the supervision of Ernest Rutherford in 1909. Important Atom Facts All …
The Atomic Theory The idea that the elements are capable of being split up into very tiny particles was rst evolved by the Greeks. The word atom comes from the greek word atomos which means indivisible and the idea that the elements are made up of atoms is called the atomic theory. A scienti c theory is a scienti c idea which has been accepted by scientists after due consider- ation. When an
Atomic Theory The History of the Atom – Student Worksheet Directions: each student in the group will answer the questions below in their own words and in complete
DUE DATE: _____ Name: _____ History of the Atom Project The atomic theory of matter is an excellent illustration of the process of science.
N B.1 The History of Atomic Theory.pdf – Google Drive Main menu
Activity – Atomic Theory Foldable . Objectives . 1. To trace the development of the atomic model. 2. To recognize the different atomic models developed. Materials . Colored Paper printed with Title Information and one sheet of white paper . Colored pencils . Handout with Discovery Timeline . Procedure – Outside of Foldable. 1. Watch teacher to see how to place the white paper on the inside
John Dalton is now called the father of modern atomic theory for his efforts. His atomic theories were introduced in 19th century England. In September of 1803, John Dalton wrote his first table of atomic weights in his daily logbook. Two years after he developed his atomic weights, he published them in a book called “A New System of Chemical Philosophy”. In it he was the first to propose
History of Atomic Theory Texas Gateway
1-Atomic-Theory-History NOTES.pdf Google Docs
A Brief History of Atomic Theory birdvilleschools.net
atomic history.pdf – The History of the Atomic Theory Fill in the boxes for each scientist who assisted the development of the atomic theory. John Dalton His Theory: A picture to describeit:.
A Brief History of Atomic Theory – Relatively Interesting
2.1 A History of Atomic Theory (Problems) Chemistry
This can be explained by atomic theory if the copper-to-chlorine ratio in the brown compound is 1 copper atom to 2 chlorine atoms, and the ratio in the green compound is 1 copper atom to 1 chlorine atom. The ratio of chlorine atoms (and thus the ratio of their masses) is therefore 2 to 1 (Figure (PageIndex{4})).
The History of the Atom Socorro Independent School District